


Product Overview
U2 Knee System は膝関節の可動における機能を回復させるために設計された Total Knee System です。
2005 年に U2 Knee System が発売されて以来、世界 39 ヵ国以上で数千件もの症例で使用されています。U2 Knee は 10 年の追跡調査において、生存率は 97.7% であり、優れた長期臨床成績を収めています。
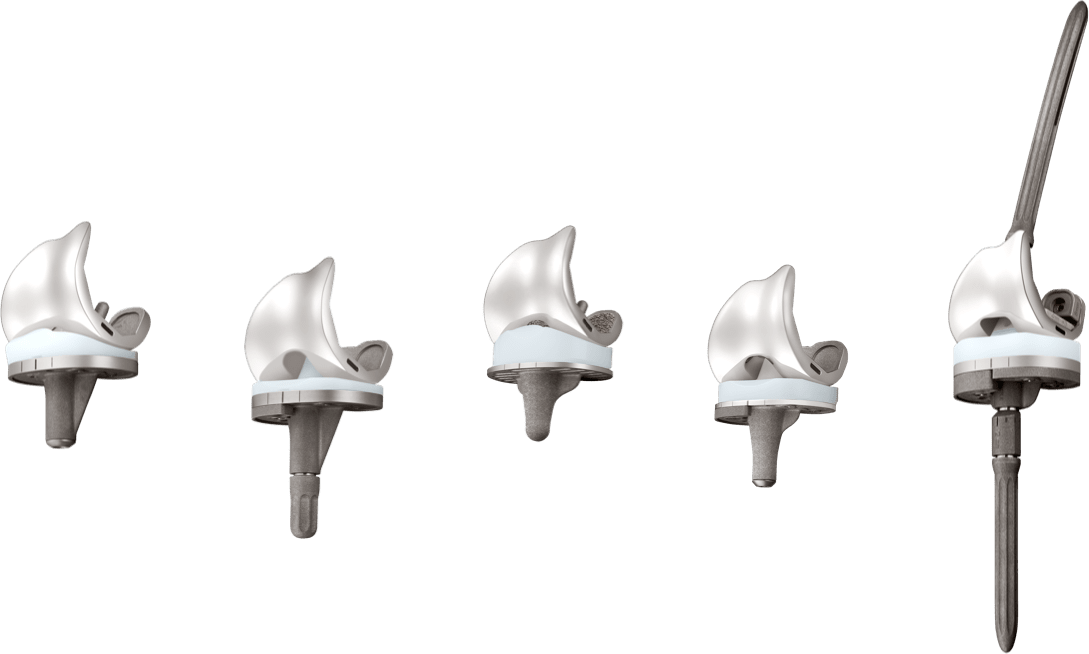
U2 Knee System は完全なサイズ互換性により、どのサイズの大腿骨コンポーネントでもどのサイズの脛骨コンポーネントと組み合わせることができます。一般的に使用される CR および PS タイプのインサートに加え、より広範囲の症例に対応するため、UC(Ultracongruent insert)を提供します。
Product Features







The U2 Knee System provides a full range of interchangeable design options with multiple insert choices with fixed and Mobile Bearing options.
For patients with severe bone deficiency, the U2 PSA Knee Revision System can be used with augments and extension stem options to manage soft tissue and bone defects.

Innovative and Award Winning Instrumentation
U2 Knee System の AiO ブロックと
MDT トライアルを使用する事により、必要な器械トレイの数を4 ケース(8 トレイ)から 1.5 ケース(3 トレイ)に減らすことが可能です。
AiO (All-In-One) Femoral Block
アンテリアリファレンス、ポステリアリファレンスのどちらのテクニックにも対応
- 1 つのブロックで 13 種類すべてのサイズにおける、前方、後方の大腿骨骨切りに対応
MDT (Modular Disposable Trials)
滅菌、洗浄コスト、および感染リスクを軽減するように設計された単回使用のトライアルセット
